
Taku Satoh, Kimio Sumaru, Toshiyuki Takagi and Toshiyuki Kanamori, Soft Matter, 2011, DOI: 10. Both photo- and thermo-responsive properties of gels have been investigated and compared.
Rates of reswelling from the light-induced shrunken state of the spirobenzopyran-functionalized gels increased with increasing ring-opening rates of spirobenzopyrans in the gels. Poly(N-isopropylacrylamide) gels were functionalized with photo-responsive spirobenzopyran and solvent/nonsolvent mixtures were used as swelling agent to introduce network heterogeneities during the gelation process. Stimuli-responsive hydrogels: Researchers have functionalised poly(N-isopropylacrylamide) gel with spirobenzopyrans and evaluated the effects of spontaneous ring-opening rates of the photo-chromic molecules on the light-responsive volume change of the subsequent gels. Keiki Kishikawa, Takahiro Inoue, Yoshiyuki Sasaki, Sumihiro Aikyo, Masahiro Takahashi and Shigeo Kohmoto, Soft Matter, 2011, 7, 7532-7538, DOI: 10.1039/C1SM05887H The slow rotation of the molecules showed unique phenomena such as no clear odd–even effect in their clearing and melting points. Thermal liquid crystal phases: An interaction assisted approach for realization of biaxiality in smectic A phases is demonstrated in addition to the effectiveness of perfluoroarene-arene and C-H/F interactions as the intermolecular interactions. The closed, colourless, hydrophobic form referred often as benzospiropyran and the open, coloured, charged merocyanine form. The triggered response of the hydrogels is discussed and a focus is placed on formulation principles, and on how the physicochemical properties of such hydrogels influence their antimicrobial/antiviral action. These gels contain both spirobenzopyran (SP) chromophores and the ruthenium catalysts that drive the oscillatory Belousov-Zhabotinsky (BZ) reaction. Variations of spiropyran molecules based on the core structure of 1,3,3-trimethylspiro chromene-2,2-indoline can exist in two distinctively different forms. As a direct evidence of the drastic photo-induced proton dissociation from the chromophores, significant pH drop from over 6 to below 4 was observed for the aqueous polymer solution in rapid response to light irradiation.Antimicrobial and antiviral hydrogels: This brief review provides some illustrative examples of different types of antimicrobial (antibacterial/antifungal) and antiviral hydrogels. Under patterned light irradiation, the polymer accumulated at the irradiated area from a uniformly dissolved state and formed micro-patterned structures based on phase transition. Further, drastic photo-induced proton dissociation and the recovery in the dark were repeated more than 10 times without resulting critical degradation. Dynamically controlled construction of microstructures was demonstrated using a spirobenzopyran-containing poly ( N -isopropylacrylamide). The p K a values of the protonated chromophores in the open-ring form, which is dominant in the dark, and closed-ring form, which is dominant under irradiation, were estimated to be 6.8 and 2.6, respectively.
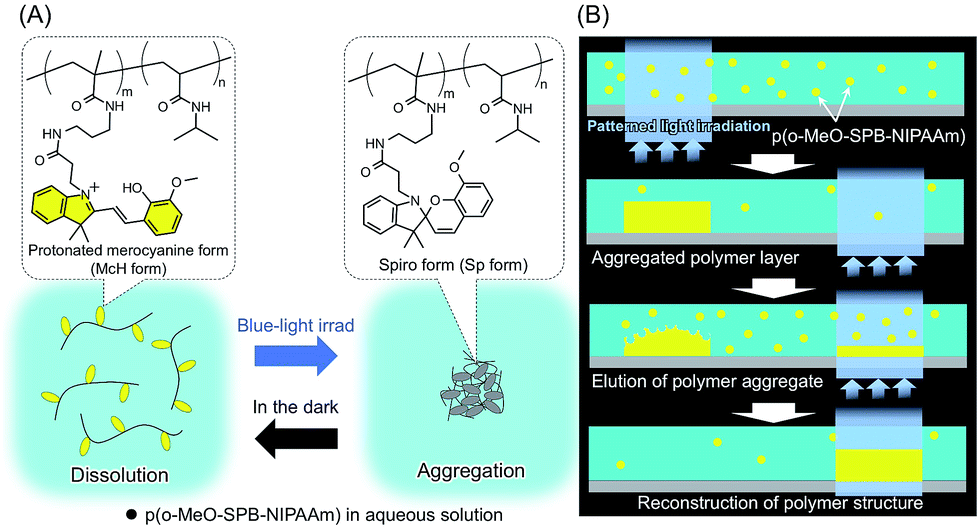
In the dark, the spirobenzopyran residue forms a ring-opened and protonated structure with showing a yellow color. From the systematic analysis using these spectra, the content ratios of the four forms of the chromophores were estimated for the conditions in the dark and under irradiation at various proton concentrations, revealing the quantitative aspect of drastic proton dissociation in response to the light irradiation. , synthesized poly(N-isopropylacrylamide) modified with spirobenzopyran and found that the aqueous solution of the copolymer exhibited remarkable dehydration by photo-irradiation under acidic condition around pH 4. The molar absorption coefficient spectra of the chromophores in open-/closed-ring forms and their protonated forms were isolated respectively from the data obtained in the various conditions of proton concentration and light irradiation. We report detailed behavior of the spirobenzopyran covalently attached to poly( N, N-dimethylacrylamide), which exhibits reversible and drastic proton dissociation in response to blue light in aqueous system.


 0 kommentar(er)
0 kommentar(er)
